SONOVEIN
No incisions
No sedation
No tumescence
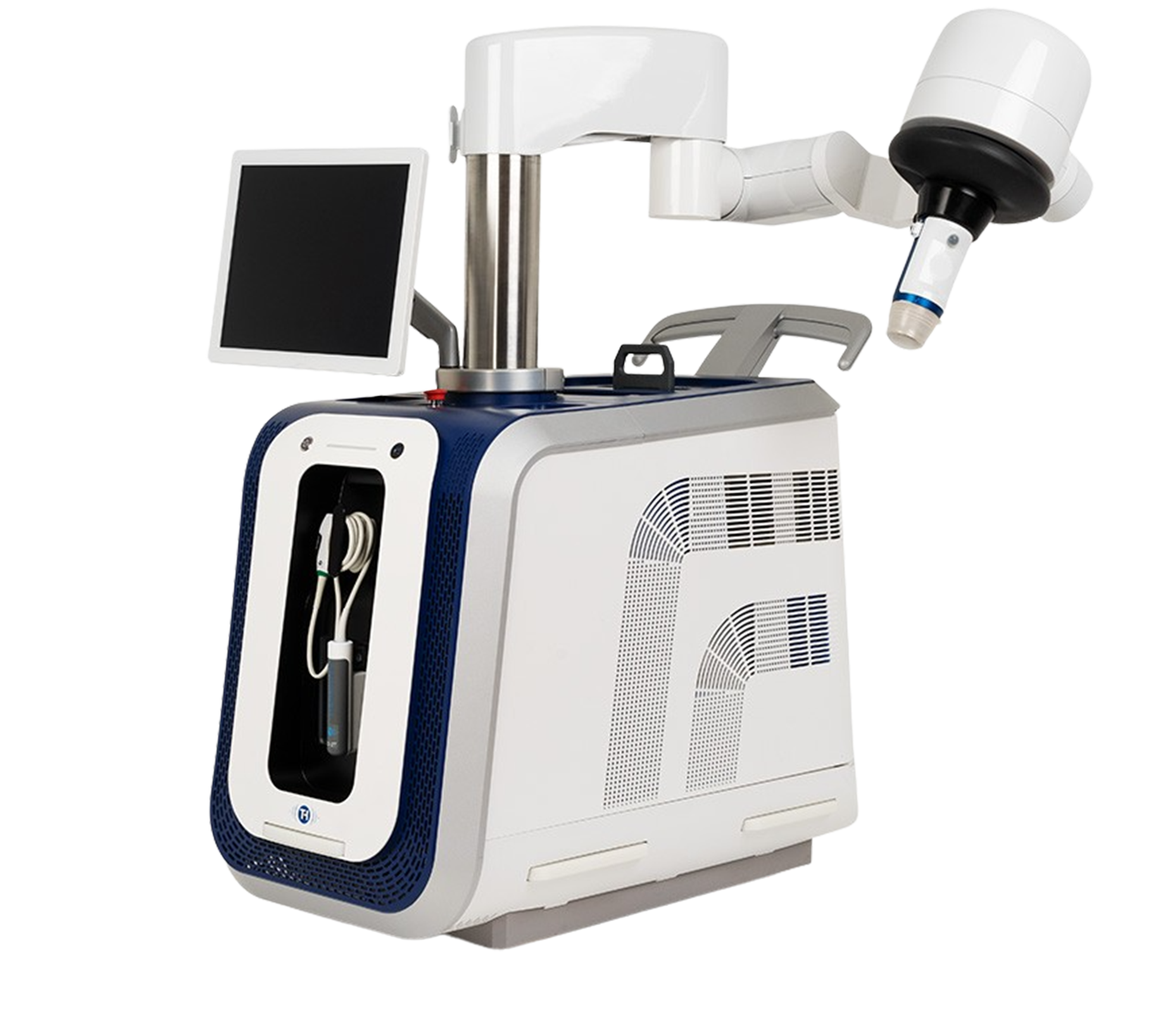
SONOVEIN® is the first and only robotic solution for the non-invasive echotherapy treatment of varicose veins:
Whiteley , HIFU for the treatment of varicose veins and venous leg ulcers, Current Medical Research and Opinion, 36:3, 509-512
From a histological perspective, vein ablation by echotherapy with SONOVEIN and radiofrequency display identical features – with the significant benefit of echotherapy being completely non-invasive.
The high-intensity ultrasound beam (HIFU) is focused on the vein and a standard ultrasound beam controls the accuracy. As the thermal energy is delivered, the vein shrinks and is sealed closed, similar to other endothermal methods – but non-invasively.
Comparison of HIFU and RF-Induced Lesions in Animal Tissues, Project Number HEG18-679, Alizée Pathology (Thurmont, USA)

C’est sensationnel, car cela ouvre de nouvelles possibilités thérapeutiques en phlébologie […] Sonovein est l’avenir.
Prof. Paolo Casoni, Expert en chirurgie vasculaire
Ces résultats démontrent que la technologie HIFU de Sonovein totalement non invasive est équivalente aux méthodes de pointe actuellement disponibles. Il s’agit assurément d’une option fiable et viable, comparable à nos traitements traditionnels, tout en offrant d’autres bénéfices aux patients.
Dr. Steve Elias, Directeur du Centre des maladies veineuses et Englewood Health Network. Cofondateur de Venous Edge et Expert Venous Management. Rédacteur en chef de AVF Vein SpecialistSONOVEIN is a class IIb medical device (according to directive 93/42/EEC) designed and marketed by Theraclion. SONOVEIN has received CE-mark for coagulation of blood vessels in patients with superficial vein reflux – CE n° 2797. Before use, read the instruction for use carefully. USA Caution-Investigational Device, Limited by Federal Law to Investigational Use.